Step-by-step explanation:
A covalent bond is defined as the bond formed due to sharing of electrons between the two chemically combining atoms.
A covalent bond always forms between two non-metals.
A non-polar covalent bond is defined as the bond in which there will be no difference in electronegativities of the combining atoms. This means that combining atoms have zero electronegativity difference.
For example,
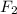 is a non-polar covalent compound as electronegativity difference of both the F atoms is zero.
is a non-polar covalent compound as electronegativity difference of both the F atoms is zero.
Thus, we can conclude that non-polar covalent bonds are always formed from atoms that are equal in electronegativity.