Answer:
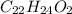 = Lipid
= Lipid
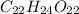 = Carbohydrate
= Carbohydrate
Step-by-step explanation:
The given compounds have molecular formulas:
Compound 1:
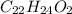
Compound 2:
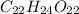
Both compounds are composed of carbon (C), hydrogen (H) and oxygen (O) with the same C:H ratios but different O content
In compound 2, the C:H:O ratio is 1:2:1 i.e. the C and O are present in equivalent amounts. This is characteristic of carbohydrates.
In compound 1, the C:H:O ratio is 1:2:(<1). The 'O' content is less than 'C' which is characteristic of lipids.