Answer:
%error = 0.802%
Step-by-step explanation:
We use the formula
%error =
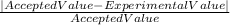 * 100
* 100
Where the accepted value is 10 mL, and the experimental value is calculated using the formula for density:
- density=mass/volume (ρ=m/V)
Experimental Volume = 10.055 g / 0.9975 g·mL⁻¹ = 10.080 mL
%error=
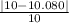 *100%
*100%
%error = 0.802%