Answer:- 7 protons and 10 electrons.
Solution:- Atomic number for nitrogen atom is 7.
For neutral atom, atomic number = number of protons = number of electrons
An ion is formed if electrons are lost or gained by the neutral atom. Here we have nitride ion where nitrogen has -3 charge. This negative 3 charge indicates the gain of 3 electrons by nitrogen atom. The number of protons remains unchanged, means there are still 7 protons.
electrons for the given ion = 7 + 3 = 10
So,
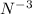 ion has 7 protons and 10 electrons.
ion has 7 protons and 10 electrons.