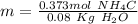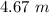Answer:
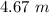
Step-by-step explanation:
If we have a 20% by weight solution indicates that in 100 g of solution we have 20 g of
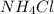 . So, in the 100 g of solution we will have 80 g of
. So, in the 100 g of solution we will have 80 g of
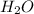 (100-20= 80). If we remember the molality equation:
(100-20= 80). If we remember the molality equation:
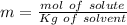
On this case the solute is
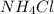 , so we have to convert from g to mol using the molar mass:
, so we have to convert from g to mol using the molar mass:
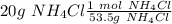
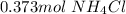
Then we have to calculate the Kg of solvent (
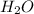 ), so:
), so:
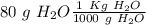
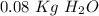
Finally, we have to divide these two values: