Answer:
37.41 grams of oxygen present in a sample of this compound that contains 50.00 grams of S.
Step-by-step explanation:
Mass of Sulfur present in the sample = 50.00 g
Mass of oxygen present in the sample = m
Total mass of the sample of the compound = = 50.00 g + m
Percentage of sulfur by mass in the compound = 57.20%
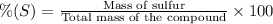
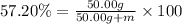
m = 37.41 g
37.41 grams of oxygen present in a sample of this compound that contains 50.00 grams of S.