Answer:
1.5 g/mL is the density of the object.
Step-by-step explanation:
Mass of an object = 7.5 g
Initial level of the water in graduated cylinder = 25.1 mL
Final level of the water in graduated cylinder = 30.1 mL
Change in volume of water level = 30.1 mL - 25.1 mL = 5.0 mL
Change in volume of water level = Volume occupied by an object
Volume of an object = 5.0 mL
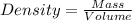
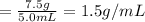
1.5 g/mL is the density of the object.