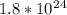Answer:- There are
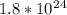 molecules of oxygen.
molecules of oxygen.
Solution:- It's two step unit conversion where grams are converted to moles and the moles are converted to molecules.
Step 1:- grams to moles-
96g x (1mol/32g) = 3 mole
Step 2:- moles to molecules-
3 mole x (6.022 x 10^23 molecule/1mole) =