Let 'a' be the number of ounces of 2%-solution in the 25-ounce mixture
and 'b' be the number of ounces of 5%-solution in the 25-ounce mixture.
Since, fluid ounces of each concentration should be combined to make 25 fl oz.
So, a+b=25 (Equation 1)
And, a container of 2% acid solution and a container of 5% acid solution should be combined to make 25 fl oz of 3.2% acid solution.
So, a of 2% + b of 5% = 3.2% of 25
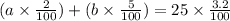
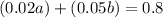
Multiplying the above equation by 100, we get
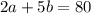 (Equation 2)
(Equation 2)
Substituting the value of a=25-b in equation 2, we get
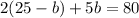
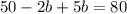
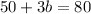
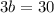
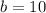
Since, a=25-b
a= 25-10
a=15.
So, 15 fluid ounces of 2% solution combined with 10 ounces of the 5% solution to create a 25-ounce mixture at 3.2% concentration of acid.