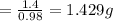Answer:
Mass of N2 required = 1.429 g
Step-by-step explanation:
The given reaction is:
N2(g) + 3H2(g) → 2NH3(g)
Mass of NH3 formed = 1.7 g
Molar mass of NH3 = 17 g/mol
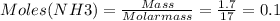
Based on the reaction stoichiometry:
1 mole of N2 forms 2 moles of NH3
Therefore, moles of N2 required to produce 0.1 moles of NH3 is:
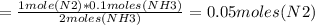
Molar mass of N2 = 28 g/mol
Mass of N2 required = moles*molar mass = 0.05*28 = 1.4 g
This is the theoretical mass corresponding to a 100% yield. Since the yield of NH3 is 98%, the corresponding mass of N2 required would be: