Answer: 374.2 K
Explanation:- Elevation in boiling point is:
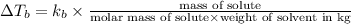
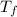 = change in boiling point
= change in boiling point
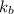 = boiling point constant=
= boiling point constant=
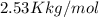
m = molality
Given: mass of solute
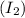 = 9.04 g
= 9.04 g
Molar mass of solute
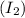 = 254 g/mol
= 254 g/mol
Weight of solvent (benzene)= 75.5 g= 0.0755 kg
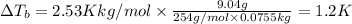
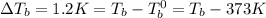
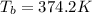
The boiling point of the solution is
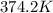 .
.