Answer : The percent yield is, 83.928 %
Solution : Given,
Experimental yield = 47 gram
Theoretical yield = 56 gram
The chemical reaction is,
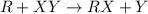
Formula used :
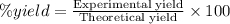
Now put all the given values in this formula, we get the percent yield.
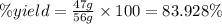
Therefore, the percent yield is, 83.928 %