Answer: A. 1.333 mol
Step-by-step explanation:
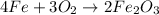
To calculate the moles, we use the equation:
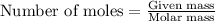
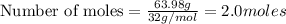
As oxygen is completely consumed, it is the limiting reagent and will limit the formation of
 .
.
According to stochiometry,
3 mole of
 produces 2 moles of
produces 2 moles of

Thus 2 moles of
 will produce=
will produce=
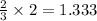 moles of
moles of

Thus the correct answer is 1.333 moles