Answer: All the elements are balanced in the given chemical equation.
Step-by-step explanation:
A balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side is equal to the total number of individual atoms on the product side. These equations follow law of conservation of mass.
The given chemical equation follows:
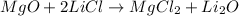
On reactant side:
Number of magnesium atoms = 1
Number of oxygen atoms = 1
Number of lithium atoms = 2
Number of chlorine atoms = 2
On product side:
Number of magnesium atoms = 1
Number of oxygen atoms = 1
Number of lithium atoms = 2
Number of chlorine atoms = 2
Hence, all the elements are balanced in the given chemical equation.