Moles of gas : 0.414
Further explanation
Given
V =29.90 liters,
P = 336.99 mmHg = 0,443 atm
T = 390K
Required
moles
Solution
In general, the gas equation can be written
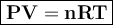
where
P = pressure, atm , N/m²
V = volume, liter
n = number of moles
R = gas constant = 0.082 l.atm / mol K (P= atm, v= liter),or 8,314 J/mol K (P=Pa or N/m2, v= m³)
T = temperature, Kelvin
Input the equation
n = PV/RT
n = 0.443.29.9/0.082.390
n= 0.414 moles