Answer:
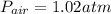
Step-by-step explanation:
Hello!
In this case, since the Dalton's law help us to realize that the total pressure of a gas mixture is computed by adding the partial pressure of each composing gas, for the mixture formed by carbon dioxide, oxygen sulfide (should be hydrogen sulfide instead) and air, we can write:
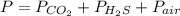
Thus, given the total pressure and the partial pressures of both carbon dioxide and hydrogen sulfide, the partial pressure of the remaining air would be:
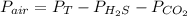
Therefore, we plug in to obtain:
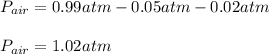
Best regards!