Answer:
0.28moles of silicon
Step-by-step explanation:
Given parameters:
Number of molecules of silicon = 1.7 x 10²³molecules
Unknown:
Number of moles of the silicon = ?
Solution:
A mole of any substance contains the Avogadro's number of particles.
1 mole of a substance contains 6.02 x 10²³molecules;
So;
1.7 x 10²³molecules contains
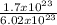 = 0.28moles of silicon
= 0.28moles of silicon