Answer:
155g
Step-by-step explanation:
To solve this problem, we have to derive the number of moles of the element given from the number of atoms first.
Number of atoms = 3.01 x 10²⁴ atoms
From mole concept;
6.02 x 10²³ atoms is found in 1 mole of a substance
3.01 x 10²⁴ atoms of P will contain
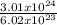 = 5moles of P
= 5moles of P
Now;
Mass of P = number of moles x molar mass
Molar mass of P = 31g/mol
Mass of P = 5 x 31 = 155g