Answer: solution b
Explanation: Formula used for Elevation in boiling point for non ionic solutes,
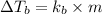
where,
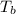 = change in boiling point
= change in boiling point
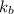 = boiling point constant
= boiling point constant
m = molality
As the molality is same for solution a and b, the change in boiling point depends on the value of boiling point constants of water and ethanol.
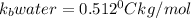 and
and
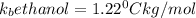
Thus solution b with higher value of
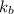 as solvent has the greatest increase in its boiling point (relative to the pure solvent)
as solvent has the greatest increase in its boiling point (relative to the pure solvent)