Answer:
6.2moles of Gold
Step-by-step explanation:
To solve this problem, we are going to use the mole concept approach.
Given that;
Number of atoms of gold is 3.73 x 10²⁴ atoms
Now;
In 1 mole of any substance, we have 6.02 x 10²³ atoms;
So;
If there 6.02 x 10²³ atoms in 1 mole of any substance;
3.73 x 10²⁴ atoms will contain
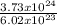 = 6.2moles of Gold
= 6.2moles of Gold