Answer:
Oxygen gas effuses at a rate that is 2.236 times that of bromine gas under the same conditions.
Step-by-step explanation:
Graham's Law:
This law states that the rate of effusion or diffusion of gas is inversely proportional to the square root of the molar mass of the gas. The equation given by this law follows the equation:
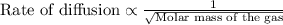
We are given:
Molar mass of oxygen gas= 32 g/mol
Molar mass of bromine gas = 160 g/mol
By taking their ratio, we get:
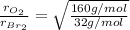
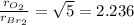
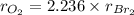
Oxygen gas effuses at a rate that is 2.236 times that of bromine gas under the same conditions.