Answer:
Solubility = 2.142 x
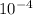 M
M
Step-by-step explanation:
Solution:
Note: This question is incomplete and lacks necessary data to solve this question. But I have found similar question on the internet and fetched the necessary data to solve:
Missing Data:
Number of Moles of N2 dissolved per litre of blood = 5.357 x
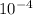 M
M
As we know that, the air is approximately. 78 mol % of N2
N2 = 0.78
Partial pressure of the N2 at 100 ft:
Partial Pressure = 0.78 x (4.0)
Partial Pressure = 3.12 atm
Now, this is 4 times the pressure of nitrogen at 1 atm air pressure.
SO,
According to the Henry's Law, we have:
Solubility = k x
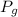
Here, k = 4 times
So,
Solubility = 4 x (5.357 x
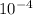 )
)
Solubility = 2.142 x
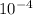 M
M