We are given with a compound, Aluminum Hydroxide (Al(OH)3), with a molar of 5.00 x
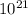
formula units. We are tasked to solve for it's corresponding mass in g. We need to solve first the molecular weight of Aluminum Hydroxide, that is
Al=27 g/mol
O=16 g/mol
H=1g/mol
Al(OH)3= 27 g/mol +16(3) g/mol +1(3) g/mol= 78 g/mol
Not that 1 mol=6.022x10^{23} formula units, hence,
5.00
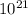
formula units x
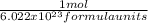
x
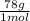
=0.648 g of Al(OH)3
Therefore, the mass of Aluminum Hydroxide is 0.648 g