Answer: 336.5 grams
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance weighs equal to the molecular mass and contains avogadro's number
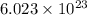 of particles.
of particles.
convert given masses into moles.
Number of Moles =
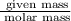
Given number of moles = 1.36
Molar mass of
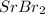 = 247.43 g/mol
= 247.43 g/mol
Putting in the values we get
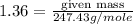
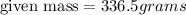
Thus mass of strontium bromide used should be 336.5 grams.