Answer: The reaction is known as synthesis reaction.
Step-by-step explanation:
Synthesis reaction is defined as the chemical reaction in which substances react in their elemental state to produce a single compound.
For Example: The formation of magnesium oxide from magnesium and oxygen gas.
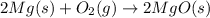
By Stoichiometry of the reaction:
2 moles of magnesium atom reacts with oxygen gas to produce 2 moles of magnesium oxide.
Hence, the reaction is known as synthesis reaction.