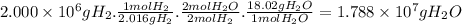Answer:
1.788 × 10⁷ g
Step-by-step explanation:
Let's consider the following balanced equation.
2 H₂ + O₂ → 2 H₂O
We can establish the following relations.
- The molar mass of H₂ is 2.016 g/mol.
- The molar ratio of H₂ to H₂O is 2:2.
- The molar mass of H₂O is 18.02 g/mol.
The mass of H₂O obtained from 200.0 kg (200.0 × 10³ g = 2.000 × 10⁶ g) of H₂ is: