Answer: The pH of the 0.02 M HCl solution is 2.
Step-by-step explanation:
The pH of the solution is defined as negative logarithm of hydrogen ion concentration in a solution.
![pH=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vwilut25e4cux34589pwoorivy6w6y51xe.png)
The pH of the HCl solution of 0.01 Molar willbe :
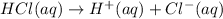
0.01 Molar of HCl will produce 0.01 M of
![[H^+]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/cky1neqovqbkiy0bd9cgh9ska0qfqez5o0.png) ions.
ions.
![[H^+]=0.01 M](https://img.qammunity.org/2019/formulas/chemistry/college/ariynjc4jc200nia5lhiwjpvqaacgomxy9.png)
![pH=-\log[H^+]=-\log[0.01]=2](https://img.qammunity.org/2019/formulas/chemistry/college/wy6bg1u437fxlg177ssmb8gu353akypwf3.png)
The pH of the 0.02 M HCl solution is 2.