Answer: Option (b) is the correct answer.
Step-by-step explanation:
It is known that energy obtained by an object due to its motion is known as kinetic energy. Whereas an energy obtained by an object due to its position is known as potential energy.
For example, when water is heated then bond between its molecules starts to break and therefore, molecules start to move as they tend to gain kinetic energy due to increase in temperature.
Also, it is known that K.E =
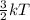
where k = boltzmann constant
T = temperature
Hence, kinetic energy is proportional to temperature. So, more is the increase in temperature more will be the increase in kinetic energy.
Thus, we can conclude that a measure of the kinetic energy of particle motion within a substance is temperature.