Answer : The correct option is, (1) Se
Explanation :
Electronic configuration : It is defined as the representation of electrons around the nucleus of an atom.
Number of electrons in an atom are determined by the electronic configuration.
Atomic number : It is defined as the number of electrons or number of protons present in a neutral atom.
(1) The given atom is, selenium (Se)
Atomic number of Se = 34
Number of electrons = 34
The electronic configuration of selenium atom is,
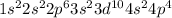
Number of valance electrons = 6
(2) The given atom is, arsenic (As)
Atomic number of As = 33
Number of electrons = 33
The electronic configuration of arsenic atom is,
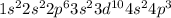
Number of valance electrons = 5
(3) The given atom is, krypton (Kr)
Atomic number of Kr = 36
Number of electrons = 36
The electronic configuration of krypton atom is,
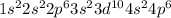
Number of valance electrons = 8
(4) The given atom is, gallium (Ga)
Atomic number of Ga = 31
Number of electrons = 31
The electronic configuration of gallium atom is,
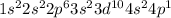
Number of valance electrons = 3
Hence, from this we conclude that the selenium element has six valence electrons in each of its atoms in the ground state.