Answer : The quantity of heat, in joules or calories, required to raise the temperature of 1 gram of a substance 1°C is known as, Specific heat capacity.
Explanation :
Heat capacity : It is defined as the heat required to raise the temperature by one degree.
Specific heat capacity : It is defined as the amount of heat absorbed by one gram of a substance to raise its temperature by one degree Celsius.
Formula used for specific heat capacity :
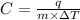
where,
C = specific heat capacity
m = mass of a substance
q = heat required
 = change in temperature of substance
= change in temperature of substance