Answer:
pH = 1.771
Step-by-step explanation:
given data
weight = 613 mg
volume of solution = 360 mL
solution
we get here first molarity that is
molarity =
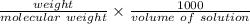 .................1
.................1
put here value
molarity =
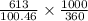
molarity = 0.01694 M
and
pH = -log[0.01694]
pH = 1.771