Step-by-step explanation:
It is known that the chemical formula of Ibuprofen is
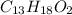 .
.
According to Avogadro, 1 mole of an atom contains
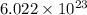 atoms.
atoms.
As molar mass of Ibuprofen is 206.29 g/mol. So, number of moles of ibuprofen will be calculated as follows.
No. of moles =
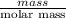
=
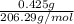
=
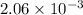 mol
mol
Since. there are 13 atoms of carbon present in a molecule of ibuprofen. Hence, total atoms of carbon present in
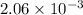 mol of Ibuprofen will be as follows.
mol of Ibuprofen will be as follows.
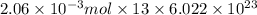
=
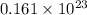 atoms
atoms
Thus, we can conclude that there are
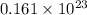 atoms of carbon are in 0.425 g of ibuprofen.
atoms of carbon are in 0.425 g of ibuprofen.