Answer:
0.124 M.
Step-by-step explanation:
Hello!
In this case, since the nickel iodide has the following formula:
NiI₂
So its molar mass is 312.5023 g/mol, in order to compute the molarity of the iodide anion, we first need the moles in 2.90 g:
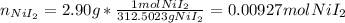
Now, since one mole of nickel(II) iodide contains two mole of iodide anions, we infer there are 0.0186 moles of iodide cations. Moreover, since the molarity is computed by dividing the moles of those ones by the volume of the solution in liters, 150 mL (0.150 L) as it does not change, it turns out:

Best regards!