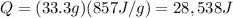Answer:
28,538 J
Step-by-step explanation:
The heat needed to vaporize the ethyl alcohol is given by:
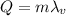
where
m = 33.3 g is the mass of the ethyl alcohol
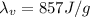 is the latent heat of vaporization of alcohol
is the latent heat of vaporization of alcohol
Since the alcohol is already at its boiling point (78 degrees), we don't need any other heat to bring it at this temperature. Therefore, we can simply calculate the heat needed by putting the numbers inside the formula: