Answer:
The value of the pressure will be 3248 kPa.
Step-by-step explanation:
Let the pressure exerted by the gas be P
The volume occupied by the gas = V = 7.5 L
Temperature of the gas = T =20°C = 293 K
(0°C = 273 K)
Number of moles of gases = 10 mol
Using an ideal gas equation:
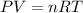
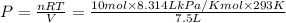
P = 3248.0026 kPa ≈ 3248 kPa
The value of the pressure will be 3248 kPa.