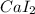Answer: d. iodine and calcium
Step-by-step explanation: Ionic bonds are formed by transfer of electrons between metal and non metals.
Covalent bonds are formed by sharing of electrons between non metals
Electronic configuration of calcium:
![[Ca]=[Ar]4s^2](https://img.qammunity.org/2019/formulas/chemistry/college/ib8sw09alv4tp2of3urdif2ag59f2yerxi.png)
Calcium atom will lose two electron to gain noble gas configuration and form calcium cation with +2 charge.
![[Ca^(2+)]={Ar]](https://img.qammunity.org/2019/formulas/chemistry/college/5nwh9urk3dvhzovizcx0zbx94q43qubaxy.png)
Electronic configuration of iodine:
![[I]=[Kr]4d^(10)5s^25p^5](https://img.qammunity.org/2019/formulas/chemistry/college/1hf55xyzpus6jd84ekdziyef4qu4tecx5l.png)
Iodine atom will gain one electron to gain noble gas configuration and form iodide ion with -1 charge.
![[I^-]=[Kr]4d^(10)5s^25p^6](https://img.qammunity.org/2019/formulas/chemistry/college/zg08ps69r6jzxpf0kfwznggh0dmn95vs1d.png)
In calcium iodide the one electron from calcium metal gets transferred to iodine atom and thus form an ionic bond to give