Answer: 0.002 M
Step-by-step explanation:
The balanced chemical equation for ionization of
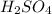 in water is:
in water is:
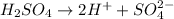
According to stoichiometry :
1 mole of
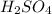 ionizes to give =2 mole of
ionizes to give =2 mole of
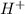 ions
ions
0.001 mole of
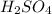 ionizes to give =
ionizes to give =
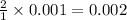 mole of
mole of
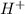 ions
ions
Thus
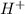 of a solution of 0.001 M aqueous sulfuric acid is 0.002 M
of a solution of 0.001 M aqueous sulfuric acid is 0.002 M