Answer: d) A:B
Explanation: Limiting reagent is the reagent which limits the formation of products.
Excess reagent is the reagent which is present in excess in the reaction.
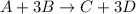
a) A:4B
As seen from the given equation, 1 mole of A reacts with 3 moles of B, thus (4-3)= 1 mole of B will remain and thus A is the limiting reagent and B is the excess reagent.
b) A:5B
As seen from the given equation, 1 mole of A reacts with 3 moles of B, thus (5-3)= 2 moles of B will remain and thus A is the limiting reagent and B is the excess reagent.
c) 2A:7B
As seen from the given equation, 1 mole of A reacts with 3 moles of B, thus 2 moles of A will react with 6 moles of B. Thus (7-6)= 1 mole of B will remain and is the excess reagent.
d) A: B
As seen from the given equation, 3 moles of B react with 1 mole of A, thus 1 mole of B will react with 0.33 moles of A and (1 - 0.33) =0.66 moles of A will remain as excess reagent. B is the limiting reagent.