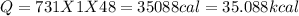Answer:
The calories required = 35088
Step-by-step explanation:
The heat required can be calculated by using specific heat of the water and mass and temperature raised.
The specific heat of water is the amount of heat required to raise the temperature of one gram of water by one degree celsius
Thus
Heat required = mass of water X specific heat X change in temperature
Given:
Specific heat of water = 1 cal /g °C
mass of water = 731 g
change in temperature = 83-35 = 48°C
Putting values