Answer : Option C) The number of neutrons present in the nucleus of each atom is 126.
Explanation :
Assuming the image as per the attachment, then the radionuclide is
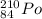 then,
then,
The element is Polonium - 210 which is an isotope of Polonium and has an atomic weight of 210
And the as given the atomic number of Polonium is 84,
Which indicates that it has 84 protons present in its nucleus and 84 electrons orbiting around its nucleus.
To calculate the number of neutrons we can simply subtract atomic weight by atomic number and get the neutrons present in its nucleus.
∴ it will be 210 – 84 = 126 neutrons
This will make the Po unstable and it will be emitting very weak gamma rays.