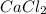Answer: The empirical formula for the given compound is
Step-by-step explanation:
We are given:
Mass of calcium = 3.609 g
Mass of chlorine = 6.384 g
To find the empirical formula of the compound, we must follow some steps:
Step 1: Converting the given masses into moles.
Moles of Ca =
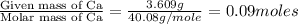
Moles of Cl =
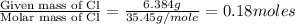
Step 2: Calculating the mole ratio of the given elements.
For the mole ratio, divide each value of moles by the smallest number of moles calculated that is 0.09
For Ca =
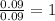
For Cl =
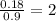
Step 3: Taking the mole ratio as their subscripts.
The ratio of Ca : Cl = 1 : 2
Hence, the empirical formula is