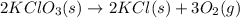Answer: 36.0 grams
Explanation: According to the law of conservation of mass, mass can neither be created nor destroyed. The total mass of the products must be same as the mass of the reactants in a balanced chemical equation.
Thus if 36 g of potassium chlorate reacts, the mass of the two products must also be equal to 36.0 g.