Answer: The correct option is b.
Explanation: To calculate the percentage yield, we use the formula:
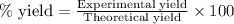 ....(1)
....(1)
For a given reaction:
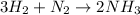
- Experimental yield calculations:
0.54 moles of ammonia is formed.
So, amount of ammonia formed will be calculated using the formula:
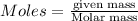 ....(2)
....(2)
Molar mass of
 = 17.031g/mol
= 17.031g/mol
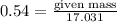
Amount of
 = 9.197g
= 9.197g
Experimental yield : 9.197 g
- Theoretical yield calculations:
By Stoichiometry of the reaction,
Here, limiting reagent is hydrogen gas because it limits the formation of product.
3 moles of hydrogen gas is producing 2 moles of ammonia
So, 2 moles of hydrogen gas will produce =
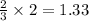 moles of ammonia.
moles of ammonia.
Amount of ammonia is calculated by using equation 2, we get:
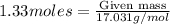
Theoretical yield of ammonia =22.65 grams
Now, putting values in equation 1, we get:
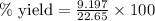
% yield=40.60 %
Hence, the correct option is b.