The correct answer is b. Since the equation is balance we can establish that A and B react in the ration 1:4. So in order for the reactants to produce more product there must exist a limiting reactant that gets used up once the ratio of reactants is 1:4 or greater than this value. The first pair of reactants gives us a 1:1 ratio, which means
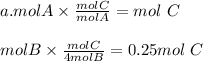 .
.
Here B is the limiting reactant and it get used up before we can go above our treshold ratio.
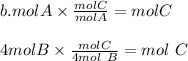
The reactants react in the ratio 1:4 which gives 1 mol of C
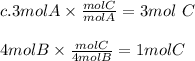
The 3:4 ratio makes B be the limiting reactant which means even though we have 3 moles of A, after 1 mol A has reacted all the 4 moles of B will be used up.
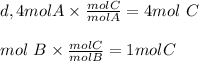
Here there is not enough moles of B to get more product.