Step-by-step explanation:
The given reaction is as follows.
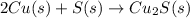
In this reaction, there are 2 atoms of copper and one atom of sulfur are reacting with each other and result in the formation of copper(I) sulfide.
Therefore, we can conclude that there is a ratio of 2:1 applies to the number of reactant atoms that enter into the reaction.