Answer:
Thorium
Step-by-step explanation:
Hello,
In this case, we have four options for the metals to be copper (63.5g/mol), cadmium (112.4g/mol), cerium (140g/mol) and thorium (232g/mol) in addition to the expected chemical reaction:
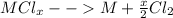
We must consider that 10.0 grams of the binary metal chloride yields 6.207 f of the pure metal, nonetheless, based on each metal's oxidation states we have seven options which are listed below:
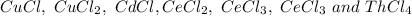
Now, a suitable strategy is to compute the metal's by mass percent in each option and compare it with the actual metal's by mass percent inferred from the statement which is 62.07% (6.207/10.0*100%). For instance, for
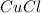 , the by mass percent of copper is:
, the by mass percent of copper is:
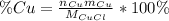
Whereas
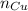 accounts for the number of atoms of copper in such compound,
accounts for the number of atoms of copper in such compound,
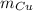 accounts for the copper's atomic mass and
accounts for the copper's atomic mass and
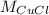 accounts for the copper (I) chloride's molar mass which is 70.9 g/mol, thus:
accounts for the copper (I) chloride's molar mass which is 70.9 g/mol, thus:
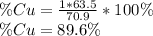
Such value does not dovetail with the percent computed from the statement (62.07%), in this manner, after doing it for all the metals, the only one that matches is the
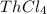 as shown below:
as shown below:
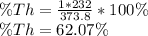
Therefore, the metal is thorium.
Best regards.