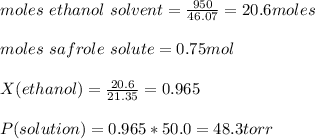Answer:
Vapor pressure of solution = 48.3 torr
Step-by-step explanation:
Given:
Moles of safrole (solute) = 0.75
Mass of ethanol (solvent) = 950 g
Vapor pressure of ethanol = 50.0 torr
To determine:
Vapor pressure of the solution
Step-by-step explanation:
Based on Raoult's Law, vapor pressure of a solution is expressed as:
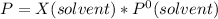
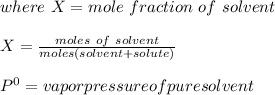
moles of