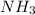Answer:
nitrogen
Step-by-step explanation:
The stoichiometry of the reaction says that
 and
and
 react 3:1 to form 2 moles of
react 3:1 to form 2 moles of
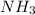 , so, if you have 3,5 moles of H, which is equal to 1,75 moles of
, so, if you have 3,5 moles of H, which is equal to 1,75 moles of
 , and 5 moles of N, which are equal to 2,5 moles of
, and 5 moles of N, which are equal to 2,5 moles of
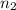 . only
. only
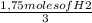 =0.583 moles of
=0.583 moles of
 , will react, leaving 1.917 moles of
, will react, leaving 1.917 moles of
 unreacted, and forming 0.583*2=1.166 moles of
unreacted, and forming 0.583*2=1.166 moles of