Answer:
The enthalpy change that would occur when 1 mole of the solute is added to 1.0000
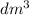 of water is 157.168 kJ/mol.
of water is 157.168 kJ/mol.
Step-by-step explanation:
Amount of ammonium chloride added = 5.350 g
Moles of ammonium chloride =
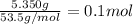
Let heat absorbed by the 0.1 mole of solute be Q.and heat lost by water be Q'.
Q = -Q'
Volume of water,V =
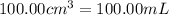
Mass of water = m
Density of water ,d= 1 g/mL
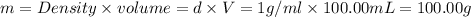
Change in temperature of the water =ΔT = 21.79°C- 25.55°C = -3.76°C
Specific heat capacity of water = c = 4.18J/g°C
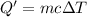
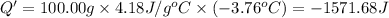
Q= -Q'=-(-1571.68 J)=1571.68 J
0.1 mole of solute absorbed 1571.68 Joules of heat from
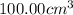 of water .
of water .
When 1 mole of solute is dissolved in
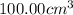 of water :
of water :
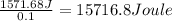
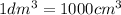
15716.8 Joule of heat is absorbed when 1 mole of solute is dissolved in
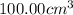 of water
of water
Heat absorbed when 1 mole of solute is dissolved in
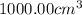 of water :
of water :
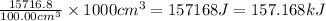
The enthalpy change that would occur when 1 mole of the solute is added to 1.0000
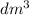 of water is 157.168 kJ/mol.
of water is 157.168 kJ/mol.