Answer: It decreases
Step-by-step explanation:
Charle's Law: This law states that volume is directly proportional to the temperature of the gas at constant pressure and number of moles.
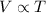 (At constant pressure and number of moles)
(At constant pressure and number of moles)
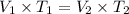
where,
 = initial volume of gas
= initial volume of gas
 = final volume of gas
= final volume of gas
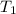 = initial temperature of gas
= initial temperature of gas
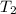 = final temperature of gas
= final temperature of gas
Therefore, as volume and temperature are proportional, if the temperature of the air in the balloon decreases, its volume also decreases.